Properties of Vinegar
What is Vinegar
"Great, the pH and other properties of vinegar
all grouped together on one convenient page!"
The physical and chemical properties of vinegar reflect the fact that vinegar is mainly a dilute aqueous solution of acetic acid.
This acid liquid which we call vinegar, is the product of two biochemical processes:
- Alcoholic fermentation, which converts natural sugars into alcohol.
- Acid fermentation in which acetobacter, microorganisms present in the air we breathe, converts the alcohol into acid.


And it is this acid, which imparts the sour taste to vinegar along with its cleaning and antiseptic or germ killing properties.
Of course most vinegars are much more than dilute solutions of acetic acid. Depending on the fruit or feed stock they are made from, and the amount of processing, they will contain various amounts of minerals, vitamins, fiber, enzymes and other organic compounds.
These are all however, minor components in the vinegar even though they are major contributors to the product's flavor and aroma as well as its overall nutrition and health benefits.
The following bulk physical and chemical properties of vinegar, such as a malt vinegar, apple cider vinegar or distilled white vinegar, come from its major components - acetic acid and water:
- The chemical formula for vinegar.
- The pH of vinegar.
- The density of vinegar.
- The freezing point of vinegar.
- The boiling point of vinegar.
- Vinegar MSDS & properties of vinegar.
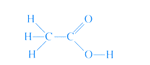
As far as chemical reactions are concerned, vinegar is a dilute solution of acetic acid, so it has the same chemical formula as acetic acid.
A molecule of acetic acid contains two carbon, four hydrogen and two oxygen atoms which is often written as CH3COOH to reflect it's actual molecular structure:
The reaction between vinegar and baking soda is used by students of all ages to learn about chemical reactions and the properties of vinegar and at the same time to have some fun launching home made rockets or demonstrating erupting volcanoes.
The term "pH" is derived from "potential hydrogen" and refers to the amount of hydrogen ions present in solution.
Mathematically, pH is equal to the negative logarithm (base 10) of the hydrogen ion concentration in moles per liter, so if the pH of a solution decreases by 1 pH unit then its hydrogen ion concentration increases by ten times.
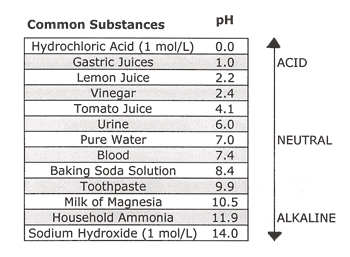
Pure water has a pH of 7 and is neutral whereas anything with a pH less than 7 is acidic and anything with a pH greater than 7 is basic.
The pH of vinegar depends upon how much acid is present, but most commercial distilled white vinegars contain 5% acetic acid and have a pH of about 2.4.
To put that in perspective, the following table compares the pH of vinegar to some other common solutions:
The density, or mass per unit volume, of a solution is used in many analytical calculations and can be measured with a hydrometer.
For a typical commercial vinegar with a 5% acetic acid content, the density is about 1.01 grams per milliliter.
The freezing point of vinegar, like the pH and density, depends on how much acetic acid is present.
A typical commercial vinegar with a 5% acetic acid content will have a freezing point of about -2 degrees Celsius (28 F).
An interesting use for the freezing point of vinegar comes up in the best selling novel "The Da Vinci Code" written by Dan Brown.
In it, he makes use of an ancient portable vault (a cryptex) to hide secret messages written on papyrus and wrapped around a fragile vial of vinegar.
The cryptex can only be opened up by dialing in the correct password. Any attempt to open it by force results in the vial breaking and the vinegar destroying the papyrus with its precious message.
Knowing that the freezing point of vinegar is only -2 degrees C, it would be a simple matter to place the cryptex in a freezer to freeze the vinegar and then to break open the vault without worrying about the vinegar destroying the papyrus and its message.
The boiling point of vinegar, like all the other physical properties of vinegar, depends on its acid concentration.
A typical distilled white vinegar containing 5% acetic acid (and 95% water) will boil at about 100.6 degrees Celsius (213 F).
Consider the acetic acid as a solute that raises the boiling point of pure water. The more acetic acid present, the higher the boiling point of the vinegar.
Pure acetic acid, called glacial acetic acid, has a boiling point of 118.1 degrees Celsius (244.5 F).
A material safety data sheet (MSDS) or safety data sheet (SDS) is a sheet containing physical and chemical information on a particular substance. It quantifies that substance's risks, safety and impact on the environment.
Everyone can use these sheets, although they are mainly designed to provide workers and emergency personnel with procedures for handling and working with various substances in a safe manner.
A safety data sheet for table strength vinegar (4.0 to 8.0% acetic acid) can be found online. (Web Link)
Each vinegar manufacturer, generally, will have his own MSDS or SDS to cover his own particular products.